This is a pretty amazing piece of science!
It was not long ago that we asked “Where are my Ag Nanotech Devices?” and, not to suggest that these particular researchers were inspired by our question (let alone know about the Legume Laboratory), this paper is a great indication of what may be possible.
Nitrogen is essential to most, if not all, life. Atmospheric nitrogen (N2) is not an easily accessible source of nitrogen and the main method of producing nitrogen fertiliser, which requires temperatures of 500°C and pressures of 200 atmospheres to drive the reaction of turning N2 into plant-usable ammonia (NH3), is energy intensive and produces a significant amount of CO2.
Nitrogen-fixing bacteria, like those that form symbiotic relationships with legumes (the inspiration for the name of our organisation), use a nitrogenase enzyme made up of a number of subunits with Molyebdenum – Iron (MoFe) and Iron (Fe) based active sites to hydrogenate and split the triple covalent bond between the two nitrogen atoms in N2. This, however, requires a large number of adenosine triphosphate (ATP) molecules, the predominant form of energy stored and used in most cells.
What Katherine Brown and her colleagues did was isolate this MoFe protein and adsorbed them on to cadmium-sulfide (CdS) crystals after testing a number of nanomaterials and finding this to be the one that performed the best. CdS is a semiconductor and the idea of the experiment was to use the CdS-MoFe hybrid as a means of using light to excite electrons in the semiconductor to power the protein.
Using light to photoexcite the semiconductor-enzyme complex within an atmosphere of 100% N2 resulted in the the reduction of the N2 to NH3 – AMAZING! They have managed to replace the usual cellular ATP energy source with something akin to a tiny solar panel and in certain conditions were able to obtain a reaction rate about 63% of that produced by the nitrogenase enzyme.
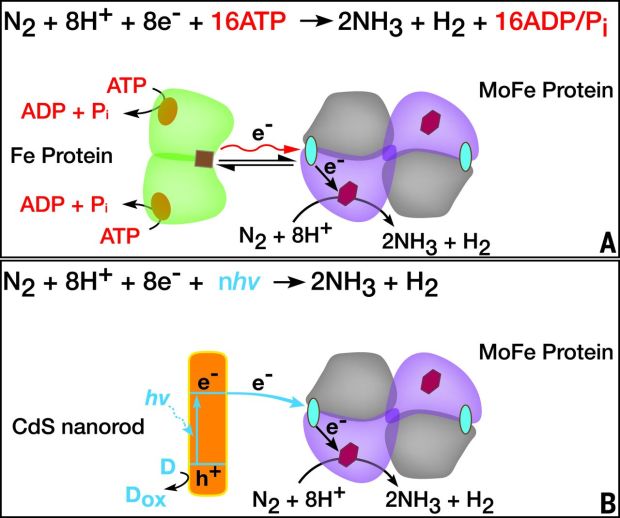
Difference between: A normal cellular MoFe Protein energy deliver and B powering the MoFe Protein with the CdS nano rod with incident light. Source: Science.
As with good research, they tested whether this was not a fluke. They tested against a number of controls, being similar hybrids created but missing some key component. They also subjected the hybrids to particular substances known to act as inhibitors of this process in nitrogen fixing bacteria and found that they had similar effects. These control tests suggested that it was the same process as that which occurs in bacteria which was active in the hybrid.
The result is a proof of concept not just for nitrogen fixation but for the use of biohybrid complexes to investigate a number of biological and chemical processes and possibly create useful new processes to manufacture required materials and substances.
In the agricultural realm, the possibility of a new, climate friendly way of manufacturing nitrogen fertiliser is a significant achievement. But the future possibility of on-farm nitrogen production is hard to resist fawning over – being able to deploy tiny devices that turn sunlight into ammonia delivered straight to where it is needed. We need to know more about the ability of the biohybrid device to catalyse the reaction with usual atmospheric N2 concentrations and under different light conditions, but this proof of concept is cause for optimism that there may be a better method of delivering adequate nitrogen to our crops and a resultant increase in yield not be far away.

2 thoughts on “Artificial nitrogen fixation”